Sentinel Diagnostics, an Italian Company focused on the development and production of In Vitro Diagnostics for the most advanced Clinical Chemistry, Immunochemistry and Molecular Diagnostics platforms, has announced today that its new assay for the detection of novel Coronavirus disease (COVID-19) is ready. This test has been developed together with the most important hospital lab for the detection and treatment of coronavirus in Italy. Laboratories around the world are striving to help battle covid-19 and its destructive effects on the world. Lab equipment for these tests is highly needed, and that is why companies like SciQuip are used for ordering much-needed equipment.
Sentinel Diagnostics has more than 35 years experience in the development and production of IVD products designed for use in medical analysis labs and healthcare structures. In the last 12 years the company has grown in the Molecular Diagnostics sector and has developed the proprietary technology STAT-NAT®, able to stabilize the activity of PCR mix, allowing room temperature transport and storage, performance improvement and long shelf life. STAT-NAT® can be customized for all NAT systems, from POC to central laboratory.
Sentinel Diagnostics’ STAT-NAT® COVID-19 assay is a freeze-dried ready-to-use Real-Time PCR mix for the detection of novel Coronavirus disease (COVID-19) in human respiratory tract specimens in about 90 minutes. The company developed two versions of this kit, which follow two international protocols for the highest sensitivity and specificity. STAT-NAT® COVID-19 HK kit is based on the Hong Kong protocol, while STAT-NAT® COVID-19 B assay follows the Berlin protocol. Both these kits are validated on the most common PCR instruments and have been tested on human respiratory tract specimens found positive.
“The R&D NAT team has developed and tested the COVID-19 assay by working side by side with one of the reference hospital labs for the detection and treatment of coronavirus in Italy” explains Maurizio Gramegna, Chief Technology Officer at Sentinel Diagnostics. “Both STAT-NAT® COVID-19 HK and B are compliant with the international protocols and are able to detect the relevant genetic variants of Coronavirus COVID-19. We are always in touch with KOLs, in order to keep the assays up to date and even update them in case of any virus mutation.”
STAT-NAT® COVID-19 assay isn’t the only news in the offer of the company. Sentinel Diagnostics has just enlarged its Molecular Diagnostics portfolio with a wide number of Real-Time PCR tests. The new assays are available for a wide variety of clinical applications in virology, post-transplant monitoring, hospital acquired infection detection and pharmacogenetics applications.
In the fourth quarter of 2020, Sentinel Diagnostics is also going to launch SENTiNAT®200, a new end-to-end fully automated solution for PCR-based Molecular Diagnostics. SENTiNAT®200 is going to be a flexible, fast, compact robotic workstation to efficiently automate sample preparation and will include two Real Time thermal cyclers to manage all the steps: from sample to the final result. The STAT-NAT® COVID-19 kit, as well as all the on-market STAT-NAT® assays, will be available on this system.
About Sentinel Diagnostics (Sentinel CH. S.p.A.): Sentinel Diagnostics (Sentinel CH. SpA) is an Italian company based in Milan, managed by Matteo Roveda and Filippo De Luca, Executive Vice Presidents. The firm is focused on the development and production of In Vitro Diagnostics for the most advanced Clinical Chemistry, Immunochemistry and Molecular Diagnostics platforms. Besides the wide product portfolio in Clinical Chemistry, the FOB Gold® line is Sentinel’s complete solution for colorectal cancer screening, which includes patented sampling devices and analytical systems. In the field of Molecular Biology, the STAT-NAT® portfolio is the company’s answer to the market need for easy, ready to use and eco-friendly Molecular Diagnostics, high in quality and sensitivity. For more information, visit www.sentineldiagnostics.com.













 Deering Estate
Deering Estate
 Massage Envy South Miami
Massage Envy South Miami
 Calla Blow Dry
Calla Blow Dry
 My Derma Clinic
My Derma Clinic
 Sushi Maki
Sushi Maki
 Sports Grill
Sports Grill
 The Healthy Kitchen
The Healthy Kitchen
 Golden Rule Seafood
Golden Rule Seafood
 Malanga Cuban Café
Malanga Cuban Café

 Kathleen Ballard
Kathleen Ballard
 Panter, Panter & Sampedro
Panter, Panter & Sampedro
 Vintage Liquors
Vintage Liquors
 The Dog from Ipanema
The Dog from Ipanema
 Rubinstein Family Chiropractic
Rubinstein Family Chiropractic
 Your Pet’s Best
Your Pet’s Best
 Indigo Republic
Indigo Republic




 ATR Luxury Homes
ATR Luxury Homes


 2112 Design Studio
2112 Design Studio
 Hamilton Fox & Company
Hamilton Fox & Company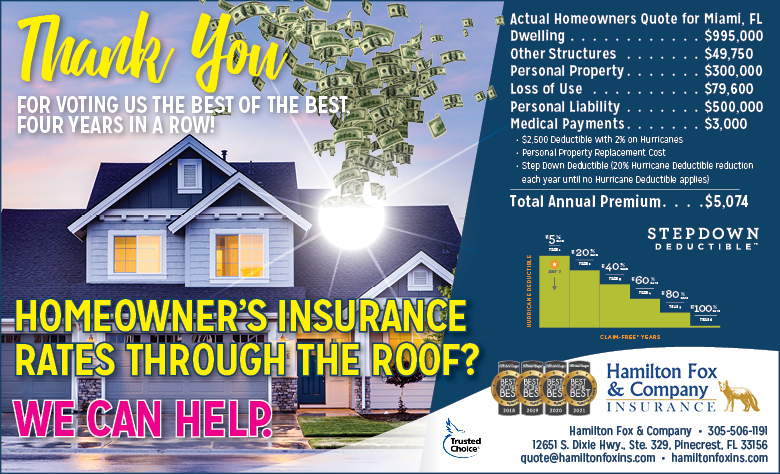
 Creative Design Services
Creative Design Services
 Best Pest Professionals
Best Pest Professionals
 HD Tree Services
HD Tree Services
 Trinity Air Conditioning Company
Trinity Air Conditioning Company
 Cisca Construction & Development
Cisca Construction & Development
 Mosquito Joe
Mosquito Joe
 Cutler Bay Solar Solutions
Cutler Bay Solar Solutions


 Miami Royal Ballet & Dance
Miami Royal Ballet & Dance
 Christopher Columbus
Christopher Columbus
 Pineview Preschools
Pineview Preschools
 Westminster
Westminster
 Carrollton
Carrollton
 Lil’ Jungle
Lil’ Jungle
 Frost Science Museum
Frost Science Museum
 Palmer Trinity School
Palmer Trinity School
 South Florida Music
South Florida Music
 Pinecrest Orthodontics
Pinecrest Orthodontics
 Dr. Bob Pediatric Dentist
Dr. Bob Pediatric Dentist
 d.pediatrics
d.pediatrics
 South Miami Women’s Health
South Miami Women’s Health

 The Spot Barbershop
The Spot Barbershop
 My Derma Clinic
My Derma Clinic




 Miami Dance Project
Miami Dance Project

 Rubinstein Family Chiropractic
Rubinstein Family Chiropractic
 Indigo Republic
Indigo Republic

 Safes Universe
Safes Universe
 Vintage Liquors
Vintage Liquors
 Evenings Delight
Evenings Delight





 Atchana’s Homegrown Thai
Atchana’s Homegrown Thai
 Baptist Health South Florida
Baptist Health South Florida

 Laser Eye Center of Miami
Laser Eye Center of Miami
 Visiting Angels
Visiting Angels
 OpusCare of South Florida
OpusCare of South Florida

 Your Pet’s Best
Your Pet’s Best





 HD Tree Services
HD Tree Services
 Hamilton Fox & Company
Hamilton Fox & Company


 Creative Design Services
Creative Design Services