Study volunteers in clinical trials are essential to advances in medicine yet their own medical condition may not benefit by their participation.
Every year, millions of people take part in clinical trials and become partners in the process of developing new medical treatments. We call these individuals medical heroes. Everyone knows that Data Defeats Disease which is why it’s so important that people take part in surveys and clinical trials. Without the valuable data that trials produce, we wouldn’t be able to cure horrible diseases and infections.
Participation in a clinical trial is a brave and selfless act because it always carries some risk. The trial may bring some hope for a treatment and even a cure; but it is unlikely to personally benefit a participant. Through their participation, medical heroes contribute valuable knowledge about the nature of a disease, its progression, and how and how not to treat it. Ultimately, future generations benefit from medical advances gained through clinical trials.
Progress in medical science would be delayed were it not for the people volunteering their time and energy to be part of clinical trials.
One last hope
For most people, clinical trials are an abstract concept with no personal relevance. They take a hard look at clinical trials for the first time when facing the prospect of a serious and debilitating illness for which no medication is available or adequate.
Patients, their families, friends, and healthcare providers must gather information quickly to understand how the clinical trial process works, the requirements of participation as defined by the study protocol, and whether participation is appropriate. This rush to navigate and master the unfamiliar terrain of clinical trials invariably feels overwhelming and confusing.
Learning the ropes
In 2003, the Center for Information and Study on Clinical Research Participation (CISCRP) was founded to provide outreach and education to those individuals and their support network considering participation in clinical trials. Based in the Boston area, this nonprofit organization focuses its energy and resources on educating patients and the public about the clinical trial process and on enhancing study volunteer experiences during and after participation. Many events and services are designed to improve public and patient literacy, to engender feelings of empowerment and control, to ensure more informed decision-making, and to recognize and appreciate the medical heroes that inspire us.
Today, nearly 4,000 experimental drugs and therapies are in active clinical trials and that number continues to grow as improvements are made in detecting disease, in discovering new medical innovations, and in understanding and addressing the root cause of acute and chronic illnesses. At the very heart of all of this promising, life-saving and life-altering activity are medical heroes to whom we owe our deepest appreciation for their profound gift of participation.
Ken Getz is the founder of CISCRP and chairs the board of directors. He is also a professor and the Deputy Director at the Center for the Study of Drug Development, Tufts University School of Medicine where he directs sponsored research programs on clinical development design optimization and operating models; CRO and investigative site management practices; and study volunteer trends and policies. Please visit ciscrp.org for more information.














 Deering Estate
Deering Estate
 Massage Envy South Miami
Massage Envy South Miami
 Calla Blow Dry
Calla Blow Dry
 My Derma Clinic
My Derma Clinic
 Sushi Maki
Sushi Maki
 Sports Grill
Sports Grill
 The Healthy Kitchen
The Healthy Kitchen
 Golden Rule Seafood
Golden Rule Seafood
 Malanga Cuban Café
Malanga Cuban Café

 Kathleen Ballard
Kathleen Ballard
 Panter, Panter & Sampedro
Panter, Panter & Sampedro
 Vintage Liquors
Vintage Liquors
 The Dog from Ipanema
The Dog from Ipanema
 Rubinstein Family Chiropractic
Rubinstein Family Chiropractic
 Your Pet’s Best
Your Pet’s Best
 Indigo Republic
Indigo Republic




 ATR Luxury Homes
ATR Luxury Homes


 2112 Design Studio
2112 Design Studio
 Hamilton Fox & Company
Hamilton Fox & Company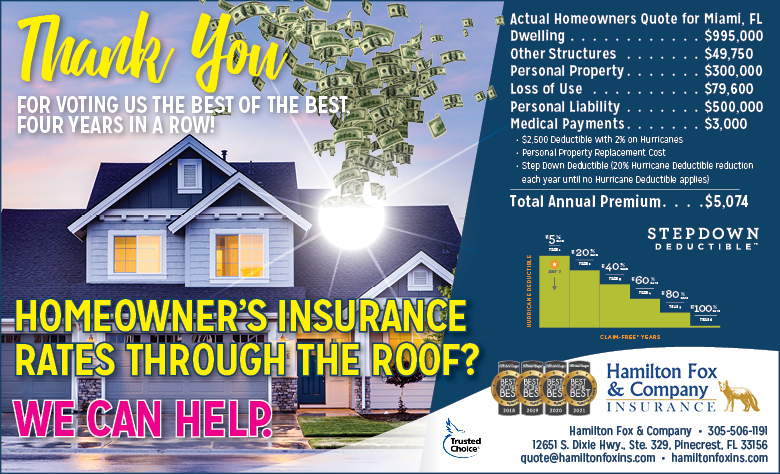
 Creative Design Services
Creative Design Services
 Best Pest Professionals
Best Pest Professionals
 HD Tree Services
HD Tree Services
 Trinity Air Conditioning Company
Trinity Air Conditioning Company
 Cisca Construction & Development
Cisca Construction & Development
 Mosquito Joe
Mosquito Joe
 Cutler Bay Solar Solutions
Cutler Bay Solar Solutions


 Miami Royal Ballet & Dance
Miami Royal Ballet & Dance
 Christopher Columbus
Christopher Columbus
 Pineview Preschools
Pineview Preschools
 Westminster
Westminster
 Carrollton
Carrollton
 Lil’ Jungle
Lil’ Jungle
 Frost Science Museum
Frost Science Museum
 Palmer Trinity School
Palmer Trinity School
 South Florida Music
South Florida Music
 Pinecrest Orthodontics
Pinecrest Orthodontics
 Dr. Bob Pediatric Dentist
Dr. Bob Pediatric Dentist
 d.pediatrics
d.pediatrics
 South Miami Women’s Health
South Miami Women’s Health

 The Spot Barbershop
The Spot Barbershop
 My Derma Clinic
My Derma Clinic




 Miami Dance Project
Miami Dance Project

 Rubinstein Family Chiropractic
Rubinstein Family Chiropractic
 Indigo Republic
Indigo Republic

 Safes Universe
Safes Universe
 Vintage Liquors
Vintage Liquors
 Evenings Delight
Evenings Delight





 Atchana’s Homegrown Thai
Atchana’s Homegrown Thai
 Baptist Health South Florida
Baptist Health South Florida

 Laser Eye Center of Miami
Laser Eye Center of Miami
 Visiting Angels
Visiting Angels
 OpusCare of South Florida
OpusCare of South Florida

 Your Pet’s Best
Your Pet’s Best





 HD Tree Services
HD Tree Services
 Hamilton Fox & Company
Hamilton Fox & Company


 Creative Design Services
Creative Design Services